Brand protection solutions that allow design and security features to live in harmony.

For the brand manager, packaging designer or packaging engineer, there are many factors to consider when designing new artwork, graphics and security for product packaging. Incorporating higher security and brand protection features into product packaging is becoming more of a priority for many companies because of the ongoing threat of counterfeiting.
However, it is challenging to mix effective brand protection, great branding and regulatory needs into one design. With different stakeholders involved, there are numerous factors affecting the perspective:
- Ease of implementation into existing packaging and print lines
- Regulatory compliance
- Brand marketing preferences
Those same stakeholders can’t ignore that the resulting impact of consumer reaction can also be critical. How can brand owners navigate these challenges and satisfy multiple stakeholders, while enabling the necessary security features and maintaining the beautiful appearance desired? Today, implementing security or brand protection solutions is possible and the impact doesn’t have to be obtrusive to your packaging medium.
Why You Can’t Ignore the Need for Security
Counterfeit operations have evolved into large, well-funded, sophisticated organizations with their tentacles in multiple countries. They survive on easy access to technology and domain expertise to compete with legitimate and other counterfeit businesses.
Companies have learned (many the hard way) that not implementing product authentication solutions and monitoring for leakage adversely affects the bottom line and potentially the health and well-being of their loyal consumers. More specifically, brand owners have discovered that adding security features on packaging can greatly mitigate:
- Lost revenue and declining market share
- Patient or consumer safety and related liability
- Recalls due to product adulteration and fraud
- Long-term erosion of consumer confidence and brand value
Counterfeiting a branded product has become easier as the supply chain expands to a global model where product visibility decreases, resulting in more opportunities for illegal activities. For years, many consumers knew of counterfeiting in terms of fake designer handbags, clothing and jewelry. Now, more consumers are becoming aware of counterfeit beauty products such as makeup, hair products, sunscreen and more. Even ‘man’s best friend’ isn’t safe from counterfeit pet food, prescriptions and over-the-counter medicines. (Source: Fake goods are not fake news, Denver Business Journal, Oct. 2018)
Flexible Security Features in Today’s Market
Product security has shifted in the last 30-35 years. In the 1980’s, the first mass deployed authentication feature, in the form of secure holography, began to show up in the industry. Early adopters were major pharmaceutical, wine/spirits and consumer electronics firms. Today, brand protection is on the radar of any company with a significant investment in high-end branded products.
Brand protection begins with the understanding that it is a process and not a product. A product undergoes the product life cycle stages: introduction, growth, maturity and decline. Brand protection as a process is a series of actions, changes and functions all directed toward the pursuit of a return on investment (ROI).
Different levels of security, varying ink technologies, and numerous covert and overt physical features can be applied to all types of packaging to challenge counterfeiters and help you stand out against your competition.
The first step of any brand protection platform is a risk assessment strategy based on the three risk dimensions: product-specific, geographic and supply chain. This then leads to solutions that match the needs recognized by that assessment.
When thinking about a multilayered approach, there are several elements to consider, starting with the type of packaging being used. The types of packaging range from flexible, barrier and decorative to promotional, rigid or dispensing. Keep in mind that no matter the type of packaging, there is an appropriate security feature to apply.
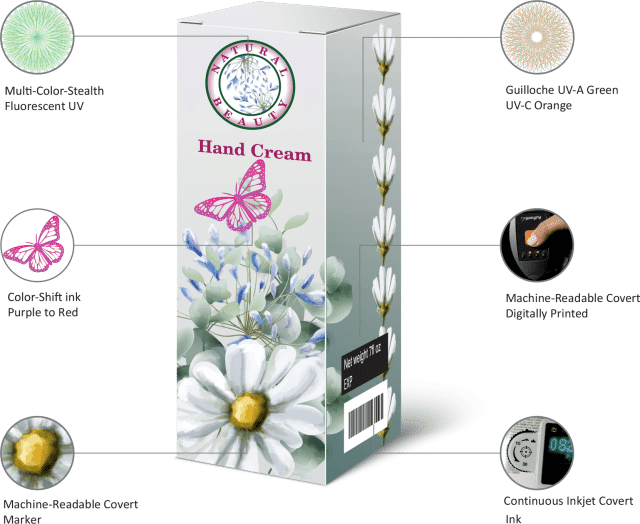
One approach in a multilayered solution is to utilize visible color-shifting inks with hidden machine-readable covert components within the ink blend (incorporate photo of our folding carton). The overt feature is a recognizable first line of defense, while the covert elements ensure evidence of authenticity. Even deeper, a molecular level marker can be added to create a forensic and court-defensible component. Most higher-security overt and covert inks are available in a wide variety of printing capabilities, including digital, offset, rotary and flexographic, and are hard to reproduce. These solutions are applicable to most substrates and customization is available in a variety of colors and coating types.
Brand Protection with Good Design—All Stakeholders Benefit
Today’s brand owners have more security choices than ever before and should realize there are effective ways to combine brand protection, great branding and regulatory needs into one design. With domain guidance and expertise, brand owners can leverage the right functional authentication feature combination that also satisfies key stakeholders within the organization. Some of the most common interests and outcomes sought by primary stakeholders are:
- Brand Protection: Desire to protect a product (brand) against counterfeiters, diversion and illicit trade. The solutions that help detect, quantify and remediate through one or more enforceable actions create a positive return on investment for the company.
- Packaging: Security solution that won’t slow down the production line or existing process. Be sure to consider the effects of implementation before selecting the solution, including graphic design, printing processes, manufacturing processes, and risk to production interruption or delays.
- Marketing: Features that don’t take away from the aesthetics of the artwork or design. The marketing department has worked hard to come up with the preferred design, color schemes and budget considerations for packaging – therefore, it’s best to only make minimal changes when adding security features to the brand.
The challenge of incorporating effective brand protection with great branding has gotten easier with the flexibility of multilayered authentication solutions. These solutions won’t jeopardize the look of the packaging; in fact, they can add to the product appeal.




 Whitepaper – Protecting Critical Care Drugs
Whitepaper – Protecting Critical Care Drugs