Counterfeit and Diverted Medicines Can Cause Severe Damage to Public Health and Brand Reputation
Understand the key considerations to implement an anti-counterfeiting strategy during the early stages of drug production to protect patients and products from unnecessary risk
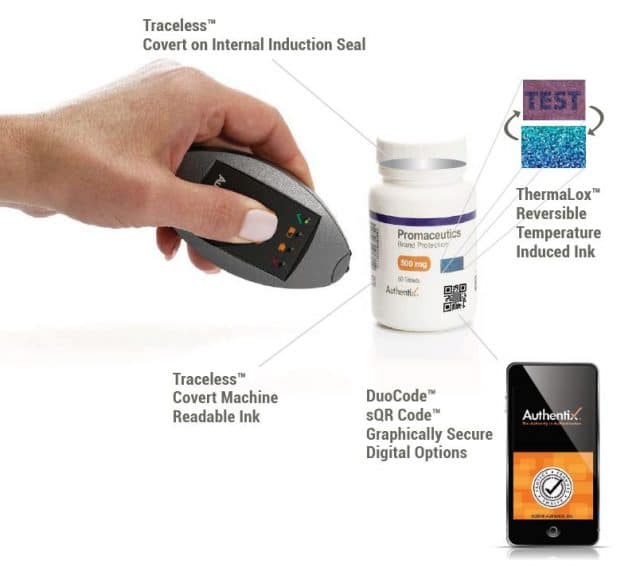
Counterfeit, adulterated and diverted medicines present a critical risk to patient safety and account for hundreds of billions of dollars in global illicit trade.
Larger companies commonly develop more advanced anti-counterfeit program (ACP) solutions in the early stages of production. Small- and mid-sized companies, however, often do not have the management bandwidth to handle the scale of these larger strategies, so leave it until later in the commercialization process when the potential risks in the market have become more known.
Waiting until the later stages of the production process to implement proper risk assessment and a product protection solution can put patients at significant risk, as well as severely and adversely impacting any company’s financial success, brand reputation and capacity to manage crisis events in the supply chain.
Protect what matters most – Customers. Brand. Revenue.
